1,
原则上来讲,只要FDA 认证的,品牌药和和仿制药都含有相同的活性成分,身体对待两者完全相同; 但是差异性可能发生在片剂或胶囊的非活性成分中,例如着色剂或填充剂等等; 还有品牌药和仿制药的配方也可能在胃中分解和溶解引起吸收的差异。
当然,这里不包括偷工减料的违规操作和不合格的生产流程
2,
品牌药贵,主要与品牌药的development 有关, including the time lapse and developmental costs.
WHY is it so expensive to develop new drugs?
- Drug development is lengthy—often taking longer than a decade.
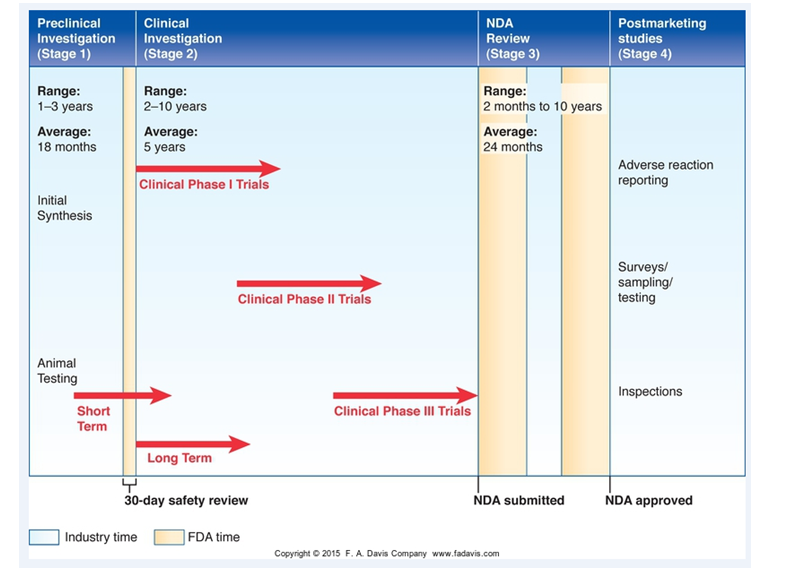
steps involved in the marketing of drugs are :
Stage 1 preclinical testing (animal models)
Stage 2 clinical testing phases I (Safety), II (Efficacy- does it work) & III (Double blind studies)
Stage 3-NDA (FDA New Drug Application)
Stage IV or post marketing surveillance.
- Drug failure rates for drugs tested in human subjects. Estimate of clinical approval success rate - 11.8%;
而仿制药少了上面的环节,because generic drug applicants do not have to repeat animal and clinical (human) studies, 当然便宜多了。这也是为甚麽仿制药的生产申请称为“abbreviated new drug application.” In fact, multiple generic companies are often approved to market a single product; this creates competition in the marketplace, typically resulting in lower prices。
3, 给个资料
Tufts study: The analysis was based, in part, on information provided by 10 pharmaceutical companies on 106 randomly selected drugs that were first tested in human subjects anywhere in the world from 1995 to 2007. “$2.6 billion figure per approved compound is based on estimated average out-of-pocket costs of $1.4 billion and time costs (expected returns that investors forego while a drug is in development) of $1.16 billion. When post-approval R&D costs of $312 million are included, the full, product lifecycle cost per approved drug, on average, rises to $2.87 billion, according to Tufts CSDD. Post-approval studies, required by the U.S. Food and Drug Administration as a condition of approval, assess new indications, new formulations, and new dosage strengths and regimens, and monitor safety and long-term side effects in patients. The Tufts CSDD estimate also accounts for expenses incurred for product development efforts that did not reach fruition.”
4, 看这个新药品牌药发展的一个图例就明白了;
不好意思, 太忙了,打中文太慢了。只好英文中文兼用。